3D Printing Medical Grade Biocompatible Materials & Products
3D Printed Cranio Maxillofacial Bone Models made with FibreTuff Medical Grade Filament
The 3D printing FibreTuff biocompatible materials as filament have a bone like surface texture, as well as appearance and radio density - vertebrae's, skulls, femurs, calcaneus, talus, metatarsal, skull cap, mandible, sacrum. Plus, FibreTuff can be sterilized with standard methods such as Autoclave.
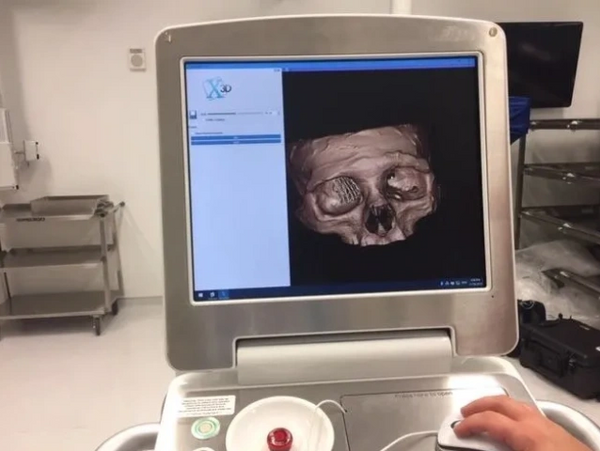
3D Printed Cranio Maxillofacial Application shows Nonbinary Radiographic Images
3D printing FibreTuff, a biocompatible polymer composite has bone like qualities including radiopacity to show device location for removal and bone bridging in CT Scans.

Annealing FibreTuff for increased strength, performance
The 3D printing FibreTuff medical grade filaments have biocompatible qualities and can be annealed to increase physical performance in some cases beyond 10%. The oven temperatures of 225F / 127C up to 45min will help increase flexibility and strength.

FibreTuff Biocompatible Compounds for 3D Printing made with ISO 13485 Certification
FibreTuff has now produced it’s biocompatible compound with ISO 13485 certification. The company will be able to produce filaments for 3D Printing to use in surgical guides and implants. FibreTuff has global recognition to produce the most advanced 3D Printed anatomical bone like models for clinical and pre surgical assessment.
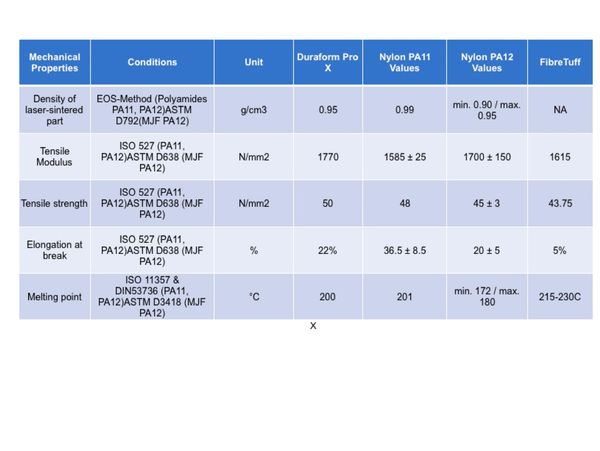
Mechanicals of FibreTuff
The specific gravity of FibreTuff I filament is 1.05 - 1.08 g/cc. This filament for 3D Printing is user friendly and doesn't require extensive drying like other highly engineered Nylon resins. FibreTuff I has proven to be biocompatible with similar properties to Nylon 11 or 12. Further, printed FibreTuff has twice the strength as bio resorbables like PCL. The FibreTuff is anisotropic, prints are stronger in the Z direction versus X and Y.
Monofilament Printing with FibreTuff
3D printing in the Z direction is an absolute for bone like construction including radio density. The cortical bone will have 100% infill and cancellous bone being less with different infill patterns available through printer manufacturer.
FibreTuff Medical Grade Filament processing for 3D Printing on Ultimaker 3 and S5
FibreTuff PAPC is biocompatible and needs to be desiccant dried for 8-10 hrs. for optimum 3D printing performance. It can be 3D Printed with various break away materials or support structure polymers to help with the vertical printing in Z direction. Standard breakaway or water soluble PVA for the Ultimaker 3D Printers can be used.
Recommended processing conditions.
The nozzle size should be .4 or .5mm with temps at 235 - 240C.
The printer bed should be heated at 80 -100C.
Cooling Fan on for first few layers than turned off
Recommended layer height .1 - .2mm
Printer oven is desired - temps 80C.
Printing speeds 45 -50 mm/sec
Density or infill is at 90 - 93% infill
Recommend Adhesive or breakaway - PVA
Please note: A new spool of FibreTuff Medical Grade filament is packaged in a vacuum sealed package with desiccant and will require some desiccant drying. If you leave spool exposed to elements over a period of time estimated at 3-4 days, moisture pickup is possible and may effect adhesion for layering. An indication of too much moisture is over extrusion or nodules present. It is recommended to desiccant dry spool for 10 hrs before printing.
FibreTuff Medical Grade Filament produced Anatomical Bone Like Models that perform like real bone
The 3D printed hand models produced at VosFox medical with FibreTuff showed details having 27 segmented bones attached with wires and pins. The process controls to make the hand included proper drying of FibreTuff before 3D printing. VosFox Medical in the Netherlands is now printing feet with each bone segmented to show realistic construction. see website www.vosfoxmedical.com

Ultimaker Material Alliance Marketplace
FibreTuff has biocompatible medical grade filaments and participates on the Ultimaker 3D Printer Materials Marketplace for 3D printing
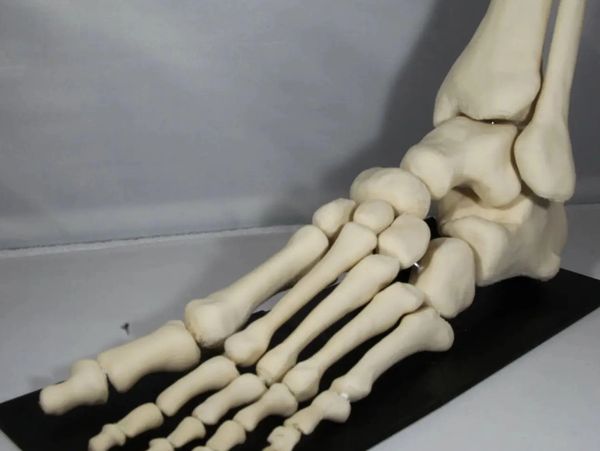
VosFox Medical is printing segmented Foot bones with FibreTuff Monofilament
VosFox Medical an established 3D printing company in Europe uses Ultimaker with FibreTuff to print biocompatible bone like replicas. These printed foot bone segments were connected with wires and glued. On the Vosfox Medical website Sandra de Vos, founder, explains how the printed foot bone was manufactured. She will sell both the bone like foot and hand models.
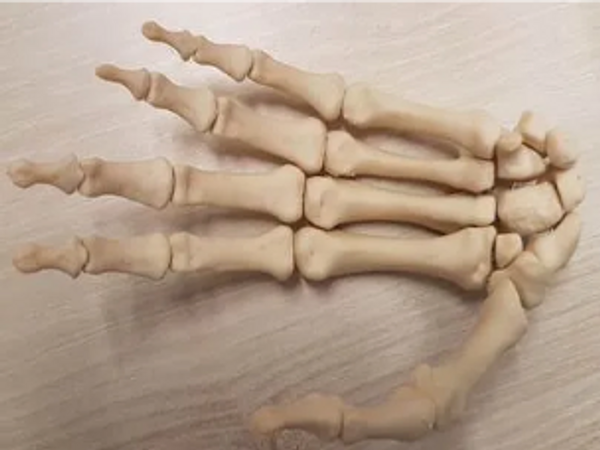
VosFox Medical Debut has printed FibreTuff hand model with 27 bones
VosFox Medical located in the Netherlands, has been 3D printing segmented bone like models to produce human hands. They have been 3D printing with Ultimaker 2+ and FibreTuff filament. The company explains the process and technique for printing the hand bone models with FibreTuff on they're website and how to purchase.
3D Printing FibreTuff PAPC Applications and Data
FibreTuff Medical Grade Monofilament prints with no offensive odors

The 3D printing of FibreTuff medical grade filaments has no offensive odors at recommended temperatures. Matter of fact, the FibreTuff is biocompatible with no maleic anhydride in some filament that can cause skin irritation.
Processing FibreTuff II on a FDM Printer

FibreTuff II medical grade filament requires a heated bed 80 -110 c . You can purchase the biocompatible FibreTuff II in a 1.75 or 2.85mm size filament. For the FibreTuff II to adhere to the heated bed a glue or adhesive sheet is required. The glue stick recommended is Cube Glue. GeckoTek is a supplier of adhesive sheet for 3D Printing. The nozzle size recommended for FibreTuff II are .5 with a processing temperature from 220 to 225C. If you use a .4 nozzle, higher heats may be necessary at 230C. A 3D Printer oven is preferred at temperatures of 80C for maximum efficiency.
Multi axis printing of FibreTuff to achieve bone like qualities
Multi axis printing of FibreTuff to achieve bone like qualities

FibreTuff has been printed with various printers and software. Additional information for 3D printing FibreTuff with more than 3 axis of freedom is available. Contact robert@fibretuff.us for more information
3D Printed filament on Ultimaker S5
Ultimaker S5 Printer had utilized nylon parameters with FibreTuff medical grade filament for 3D Printing the part shown in the video at RAPID 2019 . The FibreTuff showed remarkable adhesion that utilized cellulose fiber in the biocompatible composition.
Bioprinting FibreTuff with nScrypt BAT
Highly sophisticated 3D printing by nScrypt BAT using FibreTuff biomedical filament creates small micropore structure.
nScrypt 3D Prints a Skull Cap with FibreTuff
nScrypt has 3D Printed a skull cap with bone like qualities using biocompatible FibreTuff medical grade filament.
FibreTuff Medical Grade Filament 3D Printed on Air Wolf
The Air Wolf Axiom 3D printing FibreTuff medical grade filament at processing speed 45mm/sec to produce a calcaneus bone having radio density. The nozzle dia. .5mm at 235c temperature. The heated bed set at 100c. No oven chamber required.
FibreTuff I Medical Grade Filament
FibreTuff II Medical Grade Filament
Cookie Policy
This website uses cookies. By continuing to use this site you accept our use of cookies.
